FDA Approval for Hand sanitizer
Hand sanitizers complying with FDA OTC Monograph do not require FDA Approval. FDA Approval (NDA or ANDA) is necessary for antiseptic hand sanitizer, which does not comply with OTC Monograph.
Eligible Hand sanitizer active ingredients in FDA OTC Monograph
Benzalkonium chloride, Ethyl alcohol or Ethanol ( 60 to 95 percent), and Isopropyl alcohol (70 to 91.3 percent) are the only eligible active ingredients in OTC Monograph.
FDA Regulations for Hand Sanitizer Manufacturers
Even though the Hand sanitizer manufacturer follow the OTC Monograph, they have to comply with all other drug manufacturer requirements like
- FDA Registration
- NDC Number
- Hand sanitizer listing
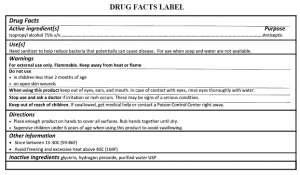
- Hand sanitizer labeling compliance
- GMP – Good Manufacturing Practice – Hand Sanitizer GMP
NDC Number for Hand Sanitizer
Each hand sanitizer must have a unique NDC number. If the difference is only in the package size, the last segment of the NDC number must be different. If the ingredient, proprietary name, intended use, etc., are different, a new NDC number must be assigned, and a separate drug listing is required.
Import requirements for Hand Sanitizer
if you are a private label distributor of hand sanitizer, you need to comply with the below requirements
- NDC Labeler code
- Hand sanitizer listing
- Hand sanitizer labeling compliance
FDA Registration Process for Hand Sanitizer
FDA registration process for hand sanitizer manufacturers includes
- DUNS number request
- Manufacturing Establishment registration
- Labeler code request for the establishment
- Drug Listing Submission with ten-digit NDC number