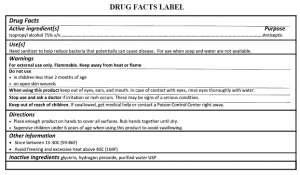
Hand Sanitizer GMP – FDA Inspection
Hand sanitizers are regulated by FDA as an OTC Monograph Drug, Contamination with Methanol (Methanol is a wood alcohol which is toxic to human body when ingested or absorbed through the skin) leads to recall of lot of hand sanitizer products from the US Market. FDA issued a country wide import alert for hand sanitizers manufactured in mexico due to lack or GMP Compliance and contamination of methanol and other toxic ingredients.
The use of alcohol-based hand sanitizer increased substantially due to COVID-19 pandemic, FDA and other agencies are more vigilant about the safety of hand sanitizer. FDA inspection for hand sanitizer is based on the GMP requirements, it is same as other OTC monograph drug products. Proper implementation of GMP will help to make sure the safety of hand sanitizer and avoid FDA waning letter after FDA inspection.
Examples of GMP Documents FDA Inspect during Hand Sanitizer Facility Inspection
- Test reports of incoming API’s which shows potency and quality attributes (absence of impurities like methanol and other toxic ingredients).
- List of all API suppliers and proof of their FDA registration.
- API supplier evaluation and approval record.
- List of equipments used to perform the hand sanitizer testing.
- Calibration records of all equipment’s used in the manufacture and testing of hand sanitizer.
- Reports of previous year’s test results.
- Certificate of Analysis (COA) from the suppliers.
- Records of frequency of lots tested.
- Stability test reports.
- List of all complaints received for hand sanitizer in current and previous years.
- Records of Corrective and preventive action (CAPA) taken for each complaint.
- Training records of employees involved in the production and testing of hand sanitizer.
LMG offers hand sanitizer GMP documentation and implementation assistance to Chicago and suburbs from Aurora, Illinois and New York & surrounding states from Manhasset, New York.